Agitators and Mixing Solutions for Lithium Processing
From the Source to Battery Grade Material

Business Development Manager
EKATO Rühr- und Mischtechnik GmbH
.
Enhance your Lithium Processing
Maximize efficiency in lithium processing with EKATO’s expertise. Reach out to our specialist for tailored solutions and innovative technologies!
Sources for Lithium Processing
Almost all lithium is currently extracted either from lithium chloride brine, which is pumped from underground, or from hard rock spodumene.
There are several different refining processes to produce battery grade Lithium, but most of them typically involve the following steps:
1.- Extraction: Lithium is extracted from brine or hard rock
2.- Concentration: The extracted lithium is concentrated through evaporation processes.
3.- Purification: The concentrated lithium undergoes purification to remove impurities, mainly by extraction, precipitation and crystallization processes.
4.- Conversion: The purified lithium is converted into lithium carbonate or lithium hydroxide by reaction or carbonation, the forms used in battery manufacturing.
In each of these stages, agitation is essential to ensure uniform mixing, proper reaction kinetics, and efficient heat and mass transfer.
Agitators and Mixing Solutions for Lithium Refining to Lithium Carbonate
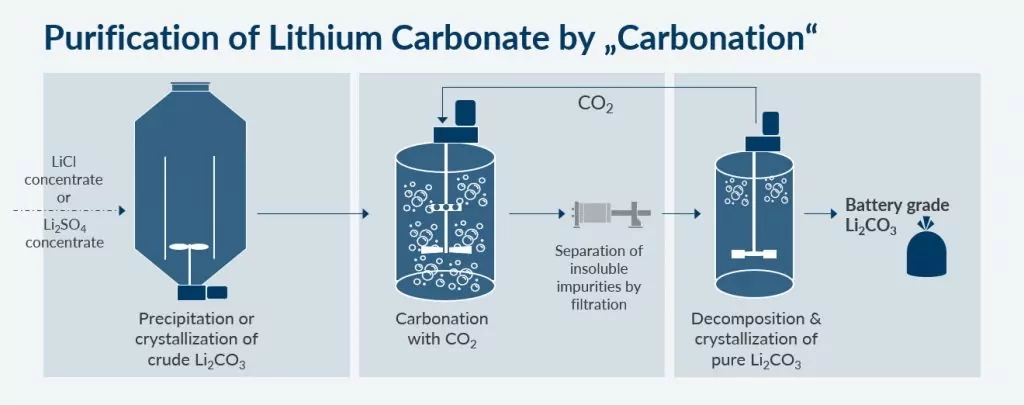
To purify Lithium Carbonate from brine or hard rock spodumene, it needs to be separated from impurities. As a first step this is done via a precipitation crystallization process. But to achieve a ready-to-process material for batteries, a further purification step is inevitable. During this step, Lithium Hydrogen Carbonate is dissolved – by adding CO2. Then the residual, solid impurities are filtered off, followed by the re-precipitation of the pure Lithium Carbonate – setting the CO2 free again.
This carbonation step involves two agitated reaction steps:
Especially gassing of the Lithium Carbonate suspension with CO2 at low temperatures should be done with an efficient gas-liquid disperser.
The decomposition at high temperatures also involves efficient mixing, here the CO2 is restored, it might be reused in the carbonization step.
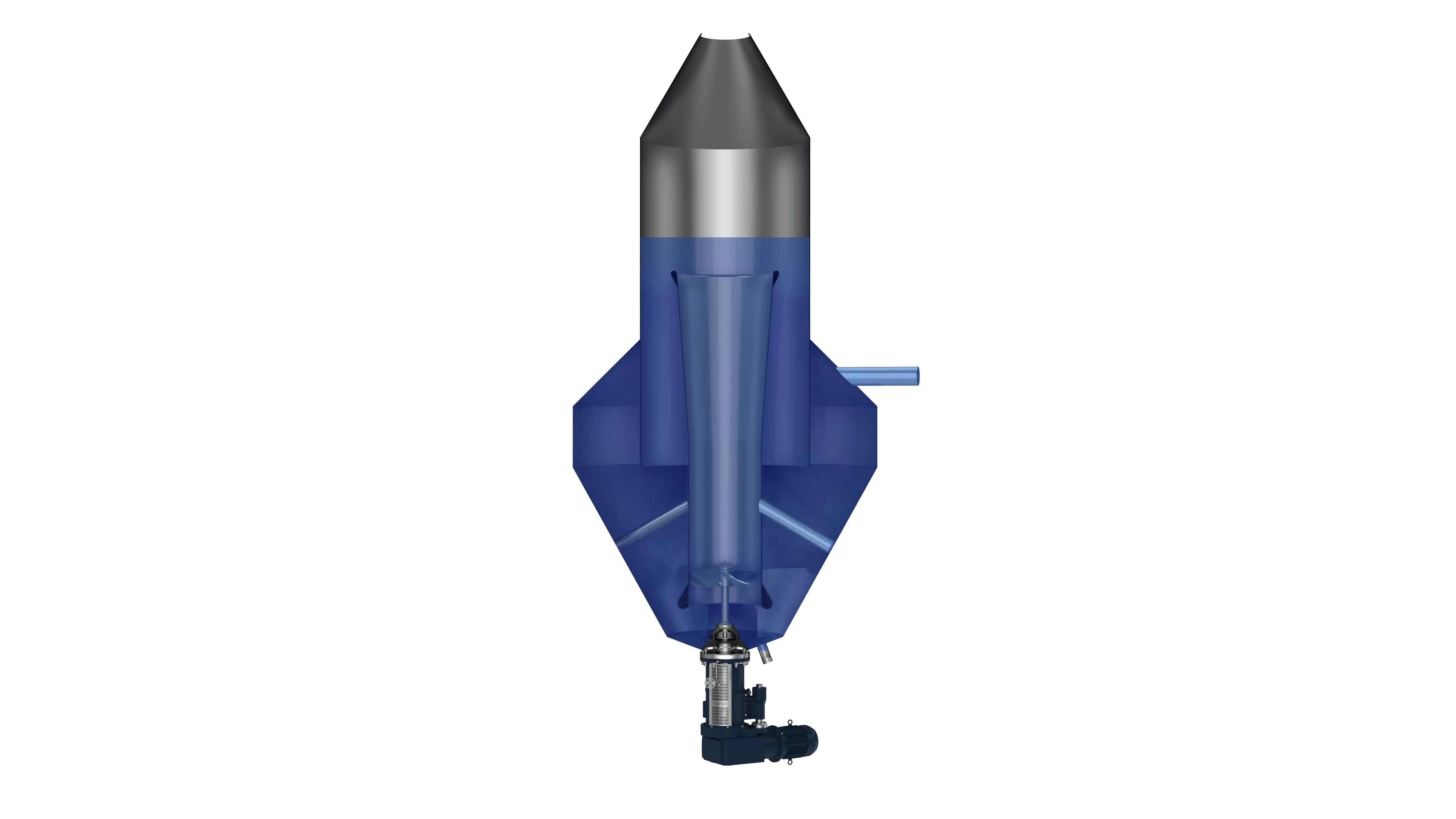
Precipitation / Crystallization Process:
The conversion of the soluble Lithium compounds (Lithium Chloride from brine, Lithium Sulfate from hard rock) to Lithium Carbonate is very often performed in draft tube crystallizers, which in this case do not work with evaporation but mostly with Sodium Carbonate Feed. On a parallel process line sodium sulphate (sodium sulphate) is usually also refined and purified in draft tube crystallizers as a side-product
Lithium Carbonate from brine:
The purified lithium chloride solution is treated with sodium carbonate (soda ash) to precipitate lithium carbonate (Li₂CO₃).
LiCl + Na₂CO₃ -> Li₂CO₃ + 2 NaCl
Lithium Carbonate from hard rock spodumene:
The lithium sulfate solution is treated with sodium carbonate to precipitate lithium carbonate.
Li₂SO₄ + Na₂CO₃ -> Li₂CO₃ + Na₂SO₄
For these processes EKATO uses its efficient axial pump the EKATO TORUSJET in the draft tube crystallizers. This solution offers various benefits:
In addition to the agitator, we can contribute to an efficient crystallizer design through laboratory tests in our own draft tube crystallizer and corresponding CFD analyses.
Carbonation / Decomposition Process:
Carbonation and decomposition are two steps in the lithium refining process that aim to remove impurities and convert the lithium into a form that can be further purified and crystallized.
Carbonation involves adding carbon dioxide or a carbonate salt to the lithium solution, which reacts with unwanted metal ions and forms insoluble precipitates. For this step a combined gassing reactor can be used, with the advantage of recycling the CO2 from the decomposition step:
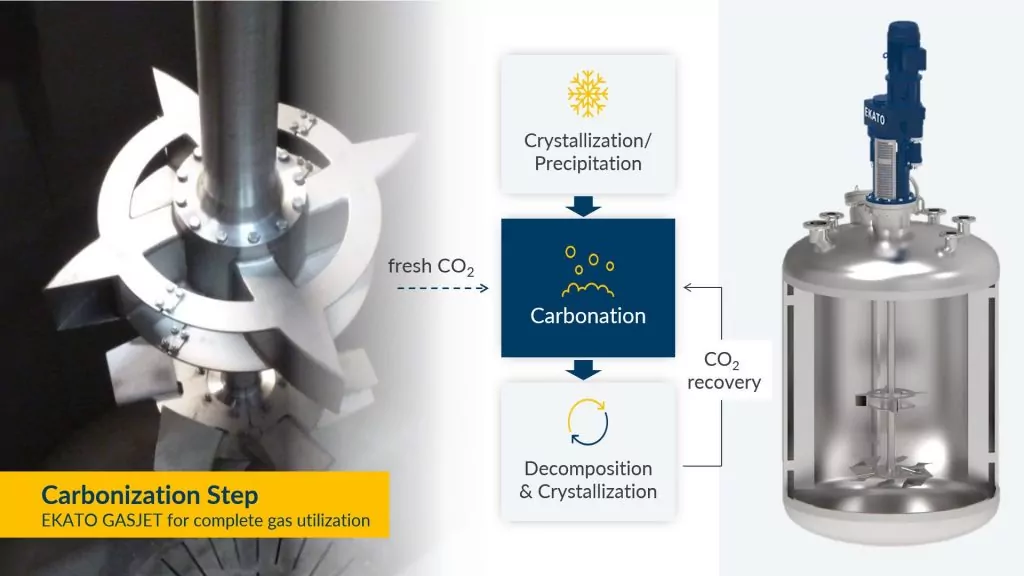
The EKATO Combined Gassing System can be adapted to almost any vessel configuration and size.
During decomposition, the solution is heated to break down any remaining soluble lithium compounds, such as sulphates or chlorides, and release lithium oxide. The lithium oxide can then be dissolved in water and neutralized with an acid to obtain a pure lithium solution. This solution can then be crystallized to obtain high-quality lithium carbonate or hydroxide. CO2 released during decomposition is recycled by returning it to the previous carbonization process where it is reacted by the EKATO Combined Gassing System.

For this decomposition and crystallization process, EKATO relies on agitators with the highly efficient EPOX-R, which offers numerous advantages:
- Very good heat transfer and heat recovery
- High local power consumption
- Efficient crystallization for high-purity lithium carbonate
- Optimized CO₂ recirculation strategies
Agitators and Mixing Solutions for Lithium Refining to Lithium Hydroxide Monohydrate
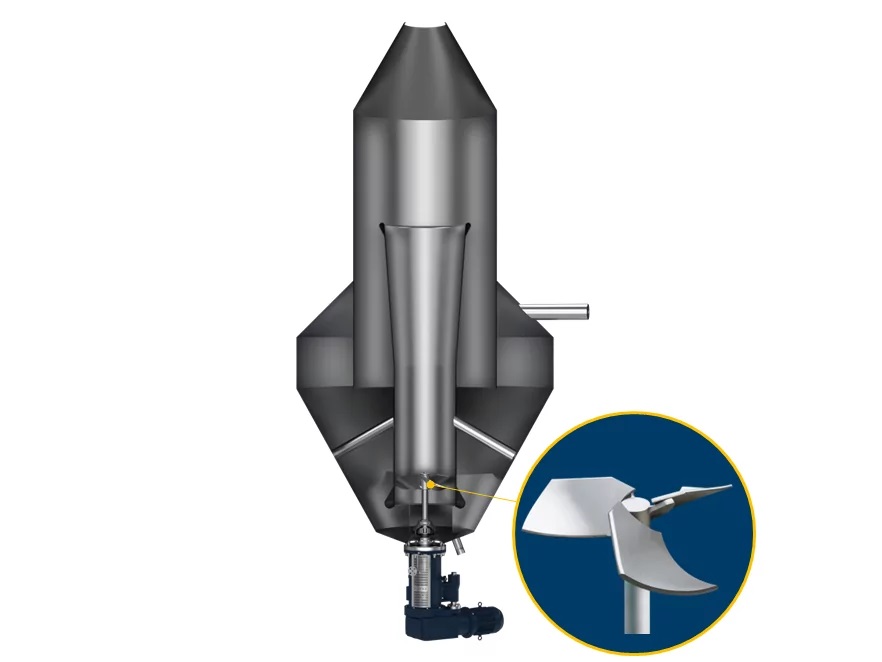
1. Concentration and primary crystallization of lithium hydroxide monohydrate
2. Purification by recrystallization
3. Final Crystallization for High Purity Lithium Hydroxide Monohydrate
Advantages of the Draft Tube Crystallizer with EKATO TORUSJET mixer
- Efficient circulation and heat transfer promote uniform crystal growth.
- Better control of crystal size and particle size distribution
- Reduced risk of fouling and scaling, resulting in more efficient and stable operation.
- Energy efficiency
Overall, the Draft Tube Crystallizer is a critical piece of equipment in the production of high quality lithium carbonate or Lithium hydroxide monohydrate that excels in its ability to provide controlled crystallization and efficient purification.
Upstream Process: Spodumene Concentrate to Lithium Sulfate
Concentration, Leaching and Neutralization
In the production of lithium carbonate or lithium hydroxide monohydrate from spodumene (hard rock), there is another important process step upstream of the precipitation and purification steps mentioned above, in which our agitators are of great importance:
The conversion of the spodumene concentrate to lithium sulphate. Here, the agitators play an essential role in leaching and the subsequent neutralization.
The roasted β-spodumene is leached with sulphuric acid (H₂SO₄) at high temperature to convert lithium into lithium sulphate (Li₂SO₄), leaving behind other minerals as insoluble residues.
beta-Spodumene + H₂SO₄ -> Li₂SO₄ + Al₂(SO₄)₃ + SiO₂
The lithium sulfate is dissolved in water to create a lithium sulfate solution.
With decades of expertise in the different leaching processes our agitators ensure efficient and powerful mixing to maintain solid suspension and enhance the leaching reaction. Our agitators can handle the very corrosive acid environment and high temperature, preventing deposits inside the vessel and ensuring uniform temperature distribution.
Battery Recycling to Regenerate Battery Grade Lithium
There will be more and more the option to regenerate lithium via EV battery recycling with hydrometallurgical processes based on active material (black mass) from recycling.
The growing demand for electric vehicles (EVs) and the limited availability of lithium resources have created an incentive for developing efficient and sustainable methods of recycling lithium from used batteries.
EKATO is one of the leading providers for customized agitators and mixing solutions in hydrometallurgical processes. This extensive expertise can be transferred and can support your chemical battery recycling project to fast success.